Introduction: Prognosis for acute myeloid leukemia (AML) patients is generally poor, with rapid progression and high probability of relapse. AML has previously been associated with high healthcare resource utilization (HRU). Changes in the AML treatment landscape may have impacts on the economic burden of AML: since 2017, seven new systemic therapies have been approved for various AML patient populations, including those unfit for intensive therapy. The frequency of hematopoietic stem cell transplantation (HSCT) has also increased over this period. This retrospective cohort study aims to characterize first-line (1L) and second-line (2L) healthcare resource utilization (HRU) and costs in newly diagnosed (ND) and relapsed/refractory (R/R) AML patients, in the context of newly available AML treatments from 2017-2022.
Methods: A large US administrative claims database (Optum) was used to identify ND AML patients between January 1, 2016, and August 31, 2022. The “ND index date” was defined as the first date a non-R/R AML diagnosis code was observed. To confirm that the initial AML diagnosis represented a true case of AML, ≥2 subsequent AML diagnoses within 60 days post-index were required. A washout period of 12 months was used to confirm no previous AML diagnosis. ND patients were required to have continuous enrollment from 1 year prior to the index until at least 30 days after the index date or death, whichever was earlier. A subgroup of R/R patients was then identified: all ND cohort members who 1) had an R/R AML diagnosis code, 2) had continuous enrollment between ND and at least 30 days after the R/R index date, and 3) did not receive 1L HSCT after the ND index date were eligible for inclusion. In this subgroup, the “R/R index date” was defined as the first date an R/R AML diagnosis code was observed. Patient per-patient-per-month (PPPM) HRU and costs were followed through the earliest of HSCT, clinical trial enrollment, death, loss of enrollment, end of data availability (September 30, 2022),or R/R diagnosis (for ND patients only). All results were reported descriptively by line of therapy and treatment received (HSCT vs. non-HSCT). Subgroup analyses were performed by age category.
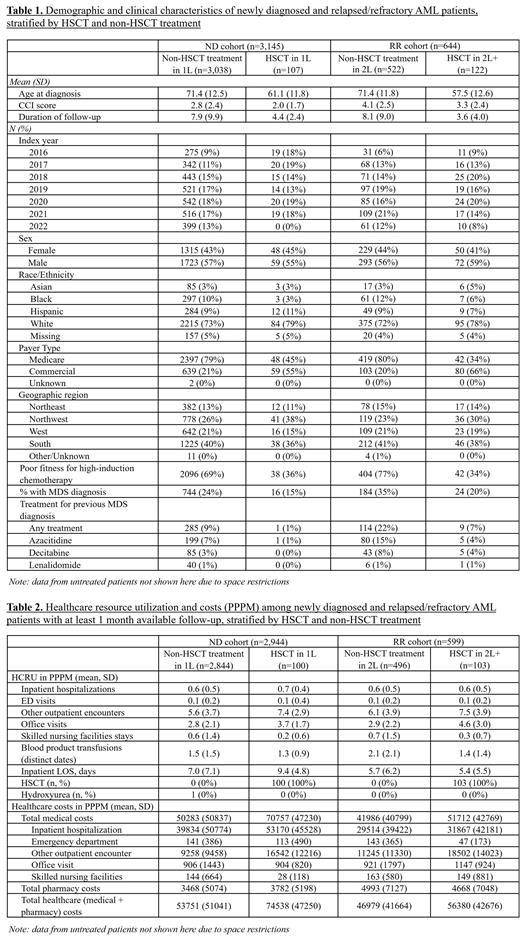
Results: We identified 3,145 ND and 644 R/R AML patients who received treatment over the study period. 3.4% of all treated ND patients received HSCT in 1L (n=107), while 19% of all treated R/R patients received HSCT in 2L (n=122). HSCT patients were younger and healthier than non-HSCT patients, reflecting selection into HSCT treatment by underlying patient characteristics (for ND patients, mean age at diagnosis 71.4 vs. 61.1; Mean Charlson Comorbidity Index (CCI) score 2.8 vs. 2.0; unfit for intensive chemotherapy 69% vs. 36%, Table 1). Patients receiving HSCT in 1L and 2L appeared to have more outpatient encounters (ND: mean 7.4 vs. 5.6; RR: mean 7.5 vs. 6.1, PPPM) than those not receiving HSCT. Number of inpatient days appeared somewhat higher among transplant recipients in 1L, but were similar to non-transplant recipients in 2L ( Table 2). While baseline pre-AML costs increased with age in 1L ($4,478 among patients 75 or older, $3,508 among patients younger than 65, PPPM), 1L follow-up costs were highest in the youngest cohort and lowest in the oldest cohort ($74,083 vs. $40,547, PPPM). Treatment-associated PPPM costs (medical + pharmacy) appeared higher in 1L vs. 2L ($54,457 vs. $48,595). When stratified by transplant status, total PPPM costs for patients receiving HSCT in 1L were higher than for those not receiving HSCT ($74,538 vs. $53,751), driven primarily by higher medical costs ($70,757 vs. $50,283). The same pattern was observed in 2L transplant vs. non-transplant patients ($56,380 vs. $46,979 total costs, $51,712 vs. $41,986 medical costs).
Conclusions: The economic burden of AML for both ND and R/R patients in the context of newly available therapies remains substantial. Total costs were comparable but slightly higher for 1L therapy as compared to 2L, and significantly higher among patients receiving HSCT compared to those not receiving HSCT in both 1L and 2L. Decreasing economic burden with age was driven by lower medical costs associated with higher rates of supportive/palliative care, suggesting a lack of available active treatment options among older AML patients.
Disclosures
Huntington:Abbvie: Consultancy; ADC Therapeutics: Consultancy; Arvinas: Consultancy; AstraZeneca: Consultancy; Bayer Healthcare: Consultancy; BeiGene USA, Inc.: Consultancy; Epizyme, Inc.: Consultancy; Genentech: Consultancy; Janssen Pharmaceuticals: Consultancy; Lilly USA, LLC: Consultancy; Merck: Consultancy; Novartis: Consultancy; Pharmacyclics LLC, An AbbVie Company: Consultancy; Seagen Inc.: Consultancy; Servier Pharmaceuticals LLC: Consultancy; TG Therapeutics: Consultancy; Tyme Inc: Consultancy. Chang:Janssen Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Fu:Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company. Loefgren:Janssen Pharmaceuticals: Current Employment. Lu:Janssen Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company.